UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): May 13, 2021
Bionano Genomics, Inc.
(Exact Name of Registrant as Specified in its Charter)
| Delaware | 001-38613 | 26-1756290 | ||||||||||||
| (State or Other Jurisdiction of Incorporation) | (Commission File Number) | (IRS Employer Identification No.) | ||||||||||||
| 9540 Towne Centre Drive, Suite 100 San Diego, California | 92121 | |||||||||||||
| (Address of Principal Executive Offices) | (Zip Code) | |||||||||||||
Registrant’s telephone number, including area code: (858) 888-7600
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||||||||
| Common Stock, $0.0001 par value per share | BNGO | The Nasdaq Stock Market, LLC | ||||||||||||
| Warrants to purchase Common Stock | BNGOW | The Nasdaq Stock Market, LLC | ||||||||||||
Item 2.02 Results of Operations and Financial Condition.
On May 13, 2021, Bionano Genomics, Inc. (the “Company”) issued a press release reporting its financial results for the first quarter ended March 31, 2021. The full text of the press release is attached as exhibit 99.1 to this Current Report on Form 8-K.
In accordance with General Instruction B.2. of Form 8-K, the information contained or incorporated herein, including the press release filed as Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, whether made before or after the date hereof, except as expressly set forth by specific reference in such filing to this Current Report on Form 8-K.
Item 8.01 Other Events.
As previously announced, the Company is hosting a conference call on May 13, 2021 at 4:30 PM Eastern Time to review its financial results for its first quarter ended March 31, 2021 and to provide a business update (the “Earnings Call”). A presentation to accompany the Earnings Call (the “Corporate Presentation”) is attached hereto as Exhibit 99.2. The Company may use information contained in the Corporate Presentation at investor and other meetings.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| Exhibit No. | Description | |||||||
| 99.1 | ||||||||
| 99.2 | ||||||||
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Bionano Genomics, Inc. | ||||||||||||||
Date: May 13, 2021 | By: | /s/ R. Erik Holmlin, Ph.D. | ||||||||||||
| R. Erik Holmlin, Ph.D. | ||||||||||||||
| President and Chief Executive Officer (Principal Executive Officer) | ||||||||||||||


Bionano Genomics Reports First Quarter 2021 Financial Results and Highlights Recent Business Progress
–179% year-over-year revenue increase driven by record sales of consumables and growth in the Saphyr installed base
–All 2021 milestones on track
–Strong balance sheet with $362 million cash as of March 31, 2021
–Conference call today, Thursday, May 13, at 4:30 pm ET
SAN DIEGO, May 13, 2021 – Bionano Genomics, Inc. (NASDAQ: BNGO) today reported financial results and business achievements for the first quarter ended March 31, 2021 and highlighted recent corporate updates.
“2021 is off to a solid start for Bionano. In the first quarter, we sold a record number of flow cells, analyzed a record number of samples in our service lab, drove broad adoption of Saphyr instruments and ramped up the installation of Saphyr systems that weren’t yet operational because of 2020’s travel restrictions,” said Erik Holmlin, PhD, CEO of Bionano. “With the largest number of Saphyr systems in service analyzing more genomes than ever before, we are seeing a sharp increase in the quality and scale of studies that are being published and presented and the awareness they build. The power of optical genome mapping, or OGM, data was on display at our own Next-Generation Cytogenomics Symposium, and through our strong presence at various key conferences in the US, Europe and China we continued to drive the awareness we believe is needed to develop the market for structural variation analysis and OGM. In Q1 2021 we saw a clear expansion into new geographical areas and into new applications such as prenatal analysis, cancer research and drug development. Our customers continued development of Saphyr-based assays for structural variation analysis in our key growth areas of prenatal and post-natal genetics, hematologic malignancies and solid tumors in Europe, the US, Canada and new markets across the world. As our customers grow the number of laboratory-developed tests (LDTs) in the market, we expect this will build out the path to reimbursement of Saphyr-based LDTs by third party payers in the US and around the world.”
Chris Stewart, Chief Financial Officer of Bionano added, “In the quarter, we solidified our foundation for growth through two successful financings, contributing to a cash position of $362 million as of the end of the quarter. We anticipate that the strength of our balance sheet will enable us to clear barriers to global adoption of OGM with Saphyr, support applications development to open up additional end-user markets and drive toward the next wave of big biology and innovation in genomics.”


Key Business Highlights
The Company executed on its commercialization strategy, continued to build scientific momentum by presenting data at key scientific meetings, and drove utilization of Saphyr® at key institutions across the globe, with the following notable announcements:
•Hosted the Next-Generation Cytogenomics symposium, which featured distinguished speakers from around the globe describing how OGM could be applied to clinical research and discovery research in pre-natal and postnatal genetic diseases, hematologic malignancies, solid tumors and host genome COVID research.
•Expanded into new geographic markets including South Africa, Greece, Russia, South Korea, and important clinical sites in the United Kingdom and Germany
◦King’s College Hospital in London and the NHS Regional Genetics Laboratory in Belfast City Hospital adopted Saphyr systems.
•Gained Saphyr adoption by the University Health Network in Toronto, which has the largest hospital diagnostic lab in Canada.
•Dr Jim Broach at Penn State University published a method for solid tumor analysis by OGM, unlocking the largest oncology market for Saphyr.
•Dr Yuval Ebenstein at Tel Aviv University published a method to analyze DNA methylation in cancer genomes, enabling a new field of cancer research and drug target discovery.
•Praxis Genomics obtained DEX Z-codes from Palmetto MolDX for billing in connection with the performance of their whole genome analysis LDT for constitutional genetic disease.
•Increased the speed of our compute on demand cloud computing solution by 30% and reduced the cost to the end-user by 50%.
•MD Anderson Cancer Center published a study showing how Saphyr significantly reduced the time to results for myelodysplastic syndrome from weeks to 4 days.
•Dr Gordana Raca from Children’s Hospital in Los Angeles presented results showing that Saphyr detects druggable gene fusions in pediatric acute leukemias that next-generation sequencing and cytogenetics missed.
•Developed new applications for Saphyr in the field of prenatal testing with publications, presentations, and the adoption of Saphyr by the Foundation for Embryonic Competence; and in cancer research and drug development with several publications on DNA methylation and DNA replication.


•Bionano customers presented a record number of presentations across all four of Bionano’s main target growth markets - prenatal, postnatal/constitutional genetics, blood cancers and solid tumor analysis – with Optical Genome Mapping (OGM) at the 2021 Annual Clinical Genetics Meeting of ACMG. We believe these publications and presentations demonstrate the utility of OGM across our targeted markets.
•Strengthened the balance sheet by raising approximately $337 million from two underwritten public offerings of shares of its common stock, the utilization of its $40 million ATM facility, and the exercise of outstanding warrants, enabling the company to advance the business without near term capital constraints.
Financial Highlights
•Total revenue was $3.2 million for the three months ended March 31, 2021, up 179% from $1.1 million in the same period of 2020. Revenue increased in all regions. The increase in product sales was driven by increased demand of our reagent rental program and consumables, while the increase in service revenue was primarily driven by sales from our Lineagen subsidiary.
•Gross margin for the first quarter of 2021 was 33%, up 8% from 25% in the same period in 2020. The increase was primarily due to a shift in our product mix toward higher margin consumables and services.
•Operating expense for the first quarter of 2021 was $12.2 million, an increase of approximately $2.2 million compared to $10.0 million for the same period in 2020. The change is primarily due to increased headcount and material and supply expense, offset by a $1M decrease in bad debt expense.
•At March 31, 2021, the Company had cash and cash equivalents of $362 million compared to cash and cash equivalents of $38.4 million at December 31, 2020. The increase in cash is due to the equity raises that were completed in January 2021.
•A gain on debt extinguishment of $1.8 million was recognized during the three months ended March 31, 2020 due to the forgiveness in full of our PPP loan, including all accrued interest.
Upcoming Milestones for 2021 – Initiatives to Drive Global Adoption of Saphyr
2Q21: Accreditation of Saphyr based LDTs for ALL & FSHD in certain EU markets
3Q21: Commercial release of prenatal assays and expansion of the menu of pediatric assays


4Q21: Interim publication of results from pediatric clinical study
4Q21: Validation of LDTs by 3 sites in both our prenatal clinical study and our pediatric clinical study
4Q21: Initial prototype of next gen high throughput Saphyr
4Q21: Reach installed base of 150 systems to achieve a 50% increase over year end 2020
Conference Call & Webcast Details
| Date: | Thursday, May 13, 2021 | ||||
| Time: | 4:30 p.m. Eastern Time | ||||
| Toll Free: | (833) 397-0959 | ||||
| International: | (614) 999-1719 | ||||
| Conference ID: | 7163806 | ||||
| Webcast: | https://edge.media-server.com/mmc/p/5hjudqv7 | ||||
To access the call, participants should dial the applicable telephone number above at least 5 minutes prior to the start of the call. An archived version of the webcast will be available for replay in the Investors section of the Bionano website.
About Bionano Genomics
Bionano is a genome analysis company providing tools and services based on its Saphyr system to scientists and clinicians conducting genetic research and patient testing, and providing diagnostic testing for those with autism spectrum disorder (ASD) and other neurodevelopmental disabilities through its Lineagen business. Bionano’s Saphyr system is a research use only platform for ultra-sensitive and ultra-specific structural variation detection that enables researchers and clinicians to accelerate the search for new diagnostics and therapeutic targets and to streamline the study of changes in chromosomes, which is known as cytogenetics. The Saphyr system is comprised of an instrument, chip consumables, reagents and a suite of data analysis tools. Bionano provides genome analysis services to provide access to data generated by the Saphyr system for researchers who prefer not to adopt the Saphyr system in their labs. Lineagen has been providing genetic testing services to families and their healthcare providers for over nine years and has performed over 65,000 tests for those with neurodevelopmental concerns. For more information, visit www.bionanogenomics.com or www.lineagen.com.


Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “may,” “will,” “expect,” “plan,” “anticipate,” “estimate,” “intend” and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) convey uncertainty of future events or outcomes and are intended to identify these forward-looking statements. Forward-looking statements include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things: the potential reimbursement of LDTs based on Saphyr; our cash position and its potential impact on our ability to execute our operational objectives; our anticipated milestones for the rest of 2021; and the advancement of our business strategy. Each of these forward-looking statements involves risks and uncertainties. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Factors that may cause such a difference include the risks and uncertainties associated with: the impact of the COVID-19 pandemic on our business and the global economy; general market conditions; changes in the competitive landscape and the introduction of competitive products; changes in our strategic and commercial plans; our ability to obtain sufficient financing to fund our strategic plans and commercialization efforts; the ability of medical and research institutions to obtain funding to support adoption or continued use of our technologies; the loss of key members of management and our commercial team; our inability to achieve the anticipated benefits from our acquisition of Lineagen; and the risks and uncertainties associated with our business and financial condition in general, including the risks and uncertainties described in our filings with the Securities and Exchange Commission, including, without limitation, our Annual Report on Form 10-K for the year ended December 31, 2021 and in other filings subsequently made by us with the Securities and Exchange Commission. All forward-looking statements contained in this press release speak only as of the date on which they were made and are based on management's assumptions and estimates as of such date. We do not undertake any obligation to publicly update any forward-looking statements, whether as a result of the receipt of new information, the occurrence of future events or otherwise.


| CONTACTS |  | ||||
| Company Contact: | |||||
| Erik Holmlin, CEO | |||||
| Bionano Genomics, Inc. | |||||
| +1 (858) 888-7610 | |||||
| eholmlin@bionanogenomics.com | |||||
| Investor Relations and | |||||
| Media Contact: | |||||
| Amy Conrad | |||||
| Juniper Point | |||||
| +1 (858) 366-3243 | |||||
| amy@juniper-point.com | |||||


| Bionano Genomics, Inc. | |||||||||||
| Condensed Consolidated Balance Sheet (Unaudited) | |||||||||||
| (Unaudited) | |||||||||||
March 31, 2021 | December 31, 2020 | ||||||||||
| Assets | |||||||||||
| Current assets: | |||||||||||
| Cash and cash equivalents | $ | 362,057,000 | $ | 38,449,000 | |||||||
| Accounts receivable, net | 1,992,000 | 2,775,000 | |||||||||
| Inventory | 3,036,000 | 3,316,000 | |||||||||
| Prepaid expenses and other current assets | 3,166,000 | 2,250,000 | |||||||||
| Total current assets | 370,251,000 | 46,790,000 | |||||||||
| Property and equipment, net | 5,806,000 | 4,910,000 | |||||||||
| Intangible Assets | 1,396,000 | 1,475,000 | |||||||||
| Goodwill | 7,173,000 | 7,173,000 | |||||||||
| Other Long Term Assets | $ | 235,000 | $ | 103,000 | |||||||
| Total assets | $ | 384,861,000 | $ | 60,451,000 | |||||||
| Liabilities and stockholders’ equity | |||||||||||
| Current liabilities: | |||||||||||
| Accounts payable | $ | 2,209,000 | $ | 2,930,000 | |||||||
| Accrued expenses | 4,659,000 | 5,599,000 | |||||||||
| Contract liabilities | 301,000 | 416,000 | |||||||||
| Total current liabilities | 7,169,000 | 8,945,000 | |||||||||
| Long-term debt, net of current portion | 14,866,000 | 16,326,000 | |||||||||
| Long-term contract liabilities | 108,000 | 98,000 | |||||||||
| Total liabilities | 22,143,000 | 25,369,000 | |||||||||
| Stockholders’ equity: | |||||||||||
| Common stock | 28,000 | 19,000 | |||||||||
| Additional paid-in capital | 516,321,000 | 178,747,000 | |||||||||
| Accumulated deficit | (153,631,000) | (143,684,000) | |||||||||
| Total stockholders’ equity | 362,718,000 | 35,082,000 | |||||||||
| Total liabilities and stockholders’ equity | $ | 384,861,000 | $ | 60,451,000 | |||||||


| Bionano Genomics, Inc. | |||||||||||
| Condensed Consolidated Statement of Operations | |||||||||||
| Three Months Ended March 31, | |||||||||||
| 2021 | 2020 | ||||||||||
| Revenue: | |||||||||||
| Product revenue | $ | 2,049,000 | $ | 983,000 | |||||||
| Service and other revenue | 1,119,000 | 153,000 | |||||||||
| Total revenue | 3,168,000 | 1,136,000 | |||||||||
| Cost of revenue: | |||||||||||
| Cost of product revenue | 1,513,000 | 774,000 | |||||||||
| Cost of service and other revenue | 612,000 | 82,000 | |||||||||
| Total cost of revenue | 2,125,000 | 856,000 | |||||||||
| Operating expenses: | |||||||||||
| Research and development | 2,678,000 | 2,674,000 | |||||||||
| Selling, general and administrative | 9,528,000 | 7,368,000 | |||||||||
| Total operating expenses | 12,206,000 | 10,042,000 | |||||||||
| Loss from operations | (11,163,000) | (9,762,000) | |||||||||
| Other income (expenses): | |||||||||||
| Interest expense | (538,000) | (761,000) | |||||||||
| Gain on debt extinguishment | 1,775,000 | — | |||||||||
| Other income (expenses) | (15,000) | 18,000 | |||||||||
| Total other income (expenses) | 1,222,000 | (743,000) | |||||||||
| Loss before income taxes | (9,941,000) | (10,505,000) | |||||||||
| Provision for income taxes | (6,000) | (5,000) | |||||||||
| Net loss | $ | (9,947,000) | $ | (10,510,000) | |||||||

May 13, 2021 Q1 2021 Webcast & Call

2 Legal Disclaimer This presentation contains forward-looking statements. Forward-looking statements describe future expectations, plans, results or strategies and are generally preceded by terms such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “potential” and similar expressions (including the negative thereof). Forward-looking statements in this presentation include, but are not limited to, statements regarding: (i) growth drivers and expected levels of our organic growth; (ii) improvements to our manufacturing cost efficiency; (iii) the impact of our investment in R&D and commercial initiatives; (iv) our ability to stay in front of competitors’ improvements in technologies; and (v) other statements that are not historical facts. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. Forward-looking statements are based only on current information, assumptions and expectations, and involve a number of risks and uncertainties relating to (i) challenges inherent in developing, manufacturing and commercializing products; (ii) the timing and mix of customer orders among our products; (iii) our ability to further deploy new products and applications and expand the markets for our technology platforms; (iv) third parties’ abilities to manufacture our instruments and consumables; (v) the success of products competitive with our own; (vi) our expectations and beliefs regarding future growth of the business and the markets in which we operate; (vii) the accuracy of our estimates, (viii) our ability to fund our operations and (ix) the application of generally accepted accounting principles which are highly complex and involve many subjective assumptions. We are under no duty to update any of these forward-looking statements after the date of this presentation to conform these statements to actual results or revised expectations, except as required by law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements contained in this presentation. More information about these and other statements, risks and uncertainties is contained in our filings with the U.S. Securities and Exchange Commission. All forward- looking statements contained in this presentation speak only as of the date on which they were made. We disclaim any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, occurrence of future events or otherwise except as required by applicable law.

3 2021: Off to a Solid Start for Bionano Q1 New applications in prenatal testing, cancer research & development 11 16 Increased speed of cloud computing solution by 30%, reducing cost to end user by 50% Talks and posters Saphyr Shipped 107 total installed base Advancing Go to Market – Bionano Data Centric Services, Reagent Rentals & Capital Purchase NEW GEOGRAPHIES Revenue: $3.2M Global Adoption of Saphyr NEW ADOPTIONS USERS DEVELOPED NEW TOOLS FOR CANCER RESEARCH, TUMOR ANALYSIS AND DRUG DEVELOPMENT Strong adoption throughout target geographies, including the US, and new markets for prenatal analysis, cancer research and drug development 33 Presentations Dr. Jim Broach Published a method for solid tumor analysis by OGM which unlocks the largest oncology market for Saphyr Dr. Yuval Ebenstein Published a method to analyze DNA methylation in cancer genomes, enabling a new field of cancer research and drug target discovery

4 Development multiple assays across a wide range of applications in human clinical research Key Steps to Widespread Global Adoption of Saphyr Publication of multiple large studies showing concordance with the existing methods A clear path for users to seek payment for OGM services they offer Goal: multiple labs in genetic disease and cancer research to develop assays and make them available for use in large studies that demonstrate the value of OGM with Saphyr

5 Bionano Data Contributes to Advances in Research ALS Resolved mosaic repeat expansions Alzheimer’s Disease Identified deletions in CR1 Leukemia In 12 patients, found dozens of novel genes with recurring structural variants Hepatocellular Carcinoma Identified Hep B Virus insertion that caused tumor and replication stress Congenital Diaphragmatic Hernia Revealed complex genome structures and new candidate genes Epilepsy and Developmental Delay Detected 90 kbp mosaic deletion in CDKL5 3q29 Microdeletion Syndrome Characterized large, complex repeats and rearrangements in parents of 3q29 patients Disorder of Sex Development Identified 6 kbp insertion in WDR11 In results expected to be published, based on comparative studies against one or more of NGS, CMAs, FISH & Karyotyping Myelodysplastic Syndrome Reduced the time to results for tumor analysis from weeks to days Pediatric Leukemia Detected druggable gene fusions that next- generation sequencing and cytogenetics missed

6 Advanced and Optimized the Performance of the Saphyr System for Adoption in Labs to Support Development of Clinical Assays and LDTs Launched an LDT for whole genome analysis with OGM for constitutional genetic disease • Developing LDTs for prenatal testing and solid tumor analysis Anticipated accreditations in EU for acute leukemias and FSHD, a genetic muscle disease

7 Lineagen Adds Revenue & Accelerates Clinical Adoption of Saphyr ESSENTIAL COMPONENTS FOR BUILDING REIMBURSED DX MENU ON SAPHYR Trained billing specialists lay groundwork for reimbursement of Saphyr LDTs COMBINING PRODUCT- AND SERVICE-BASED BUSINESSES Proprietary database from Toronto Sick Kids Hospital provides basis for differentiated tests, current and future +60,000 tests performed on +30,000 patients, and counting Proprietary Content in Pediatric Neuro Developmental Disorders Patient Samples & Database Provide expertise for improved Saphyr Dx and workflow integration Differentiated service and critical link between physicians, patients and families Leverage existing relationships and contracts with payors Clinical Cytogeneticists & Custom Interpretation Software Genetic Counseling Certified Coders 3rd Party Payor Contracts REVENUE: IMMEDIATE IMPACT Lineagen adds new revenue streams to help support growth of the combined business Provides centralized and decentralized offerings to support the broader market • Development of proprietary LDTs helps drive Saphyr adoption • Enhances Bionano offering to pharma customers seeking new therapeutic targets CLIA Certification Accelerates Saphyr entry into the clinic by adding content, skills, expertise and sample archives

Clinical Affairs Summary Dr. Alka Chaubey CMO achaubey@bionanogenomics.com
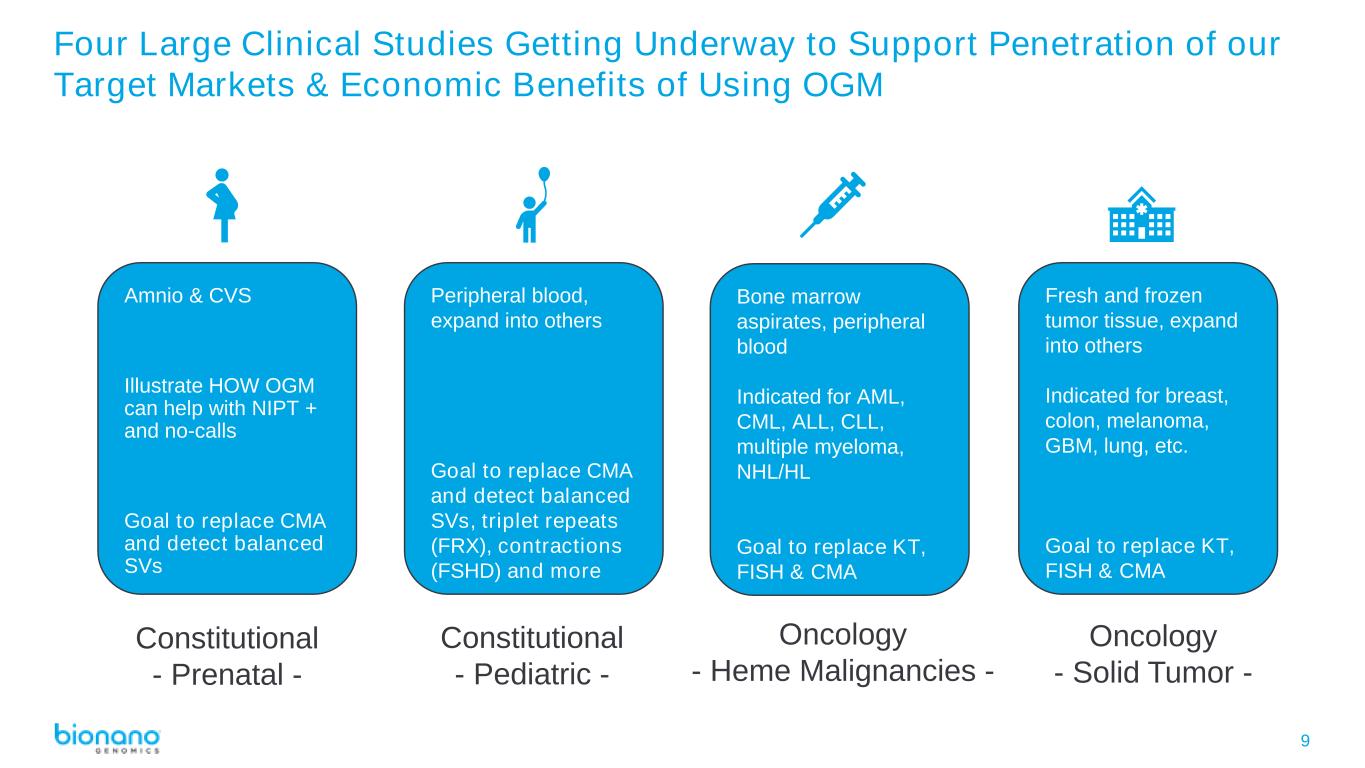
9 Four Large Clinical Studies Getting Underway to Support Penetration of our Target Markets & Economic Benefits of Using OGM Constitutional - Prenatal - Constitutional - Pediatric - Oncology - Heme Malignancies - Oncology - Solid Tumor - Amnio & CVS Illustrate HOW OGM can help with NIPT + and no-calls Goal to replace CMA and detect balanced SVs Peripheral blood, expand into others Goal to replace CMA and detect balanced SVs, triplet repeats (FRX), contractions (FSHD) and more Bone marrow aspirates, peripheral blood Indicated for AML, CML, ALL, CLL, multiple myeloma, NHL/HL Goal to replace KT, FISH & CMA Fresh and frozen tumor tissue, expand into others Indicated for breast, colon, melanoma, GBM, lung, etc. Goal to replace KT, FISH & CMA

Financial Overview Chris Stewart CFO cstewart@bionanogenomics.com

11 Q1 2021 – Financial Results and Key Highlights • Total revenue was $3.2M, up 179% from Q1 2020 • Year on year, revenue was up in all geographies and across both product and service revenue • Raised $337M from two underwritten public offerings, utilization ATM facility and exercise of outstanding warrants Strong cash position with $362M to end Q1 2021 $M, except EPS 1Q21 Revenue $3.2 Cost of Revenue 2.1 Gross Profit 1.0 Gross Margin % 33% Operating Expense 12.2 Operating Income (Loss) (11.2) Other Expense (1.2) Net Income (Loss) ($9.9) Weighted Avg Shares (M) 264 Earnings per Share ($0.04) Cash $362

Summary R. Erik Holmlin CEO eholmlin@bionanogenomics.com

13 • Commercial release of prenatal assays for Saphyr and expansion of Saphyr’s menu of pediatric assays Key Anticipated Milestones Expected to Drive Value for BNGO 3Q • Accreditation of Saphyr based LDTs for ALL & FSHD in certain EU markets 2Q 4Q • Interim publication of results from pediatric clinical study • Validation of 3 LDTs total by sites in our prenatal and pediatric clinical studies • Prototype of next-gen high throughput Saphyr • Targeted installed base of 150 systems, potentially a 50% increase over year end 2020 2021

Questions & Answers Contact: R. Erik Holmlin CEO eholmlin@bionanogenomics.com Chris Stewart CFO cstewart@bionanogenomics.com
